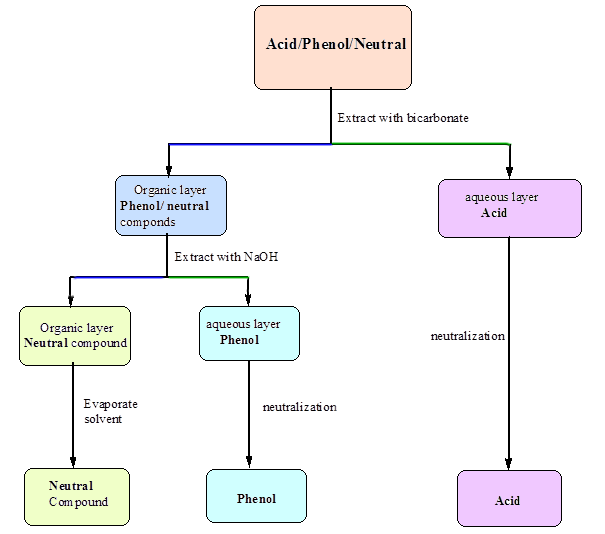
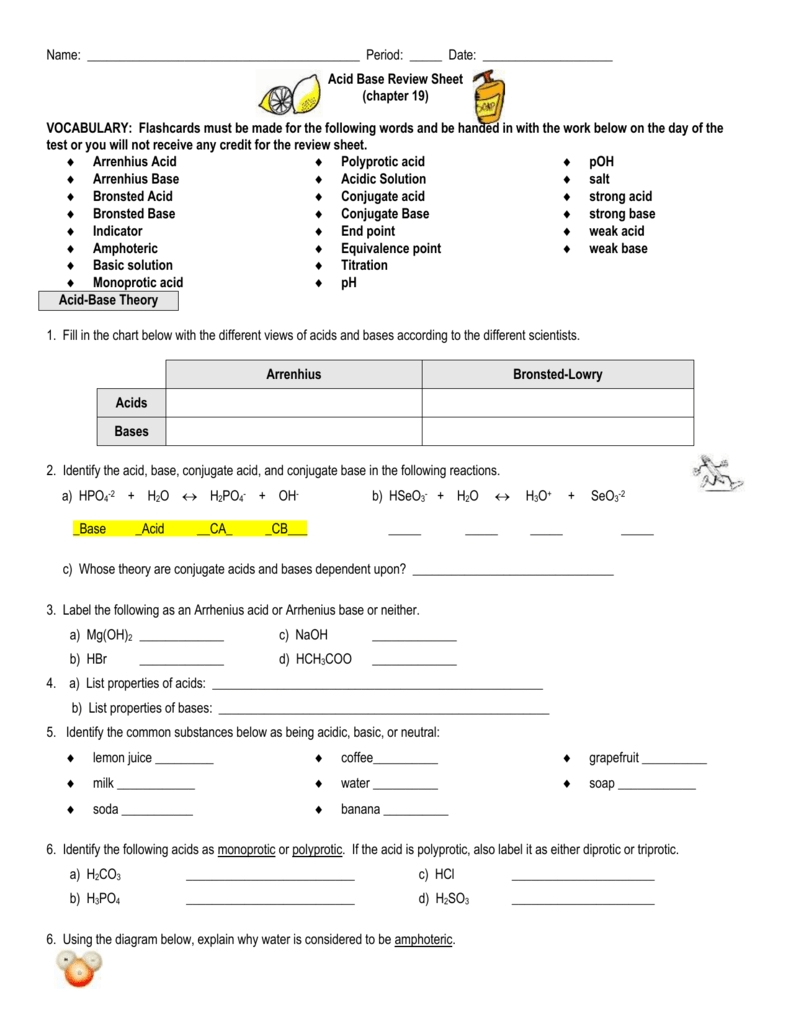
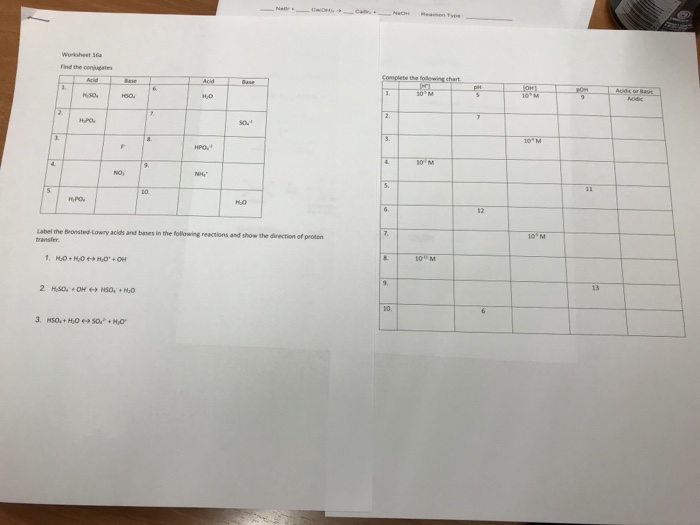
Acids And Base Chart
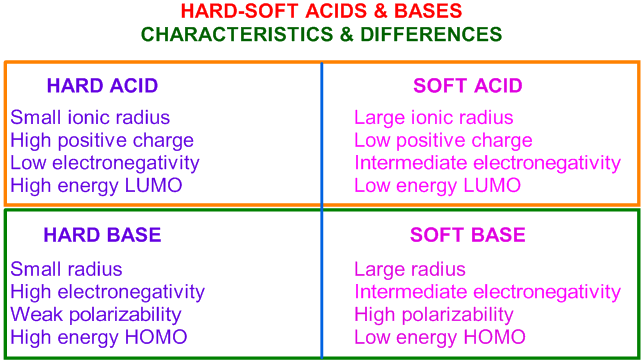
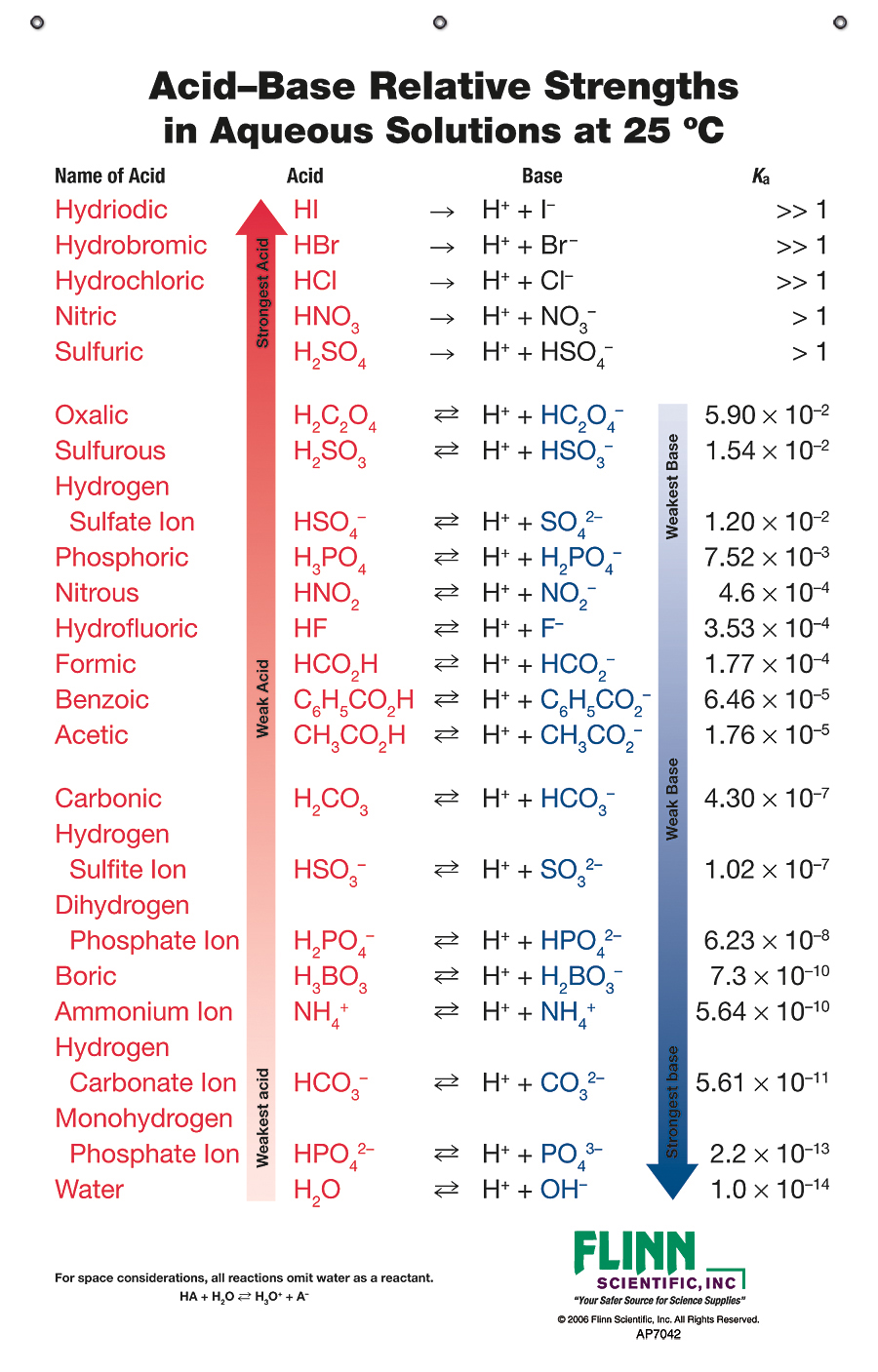
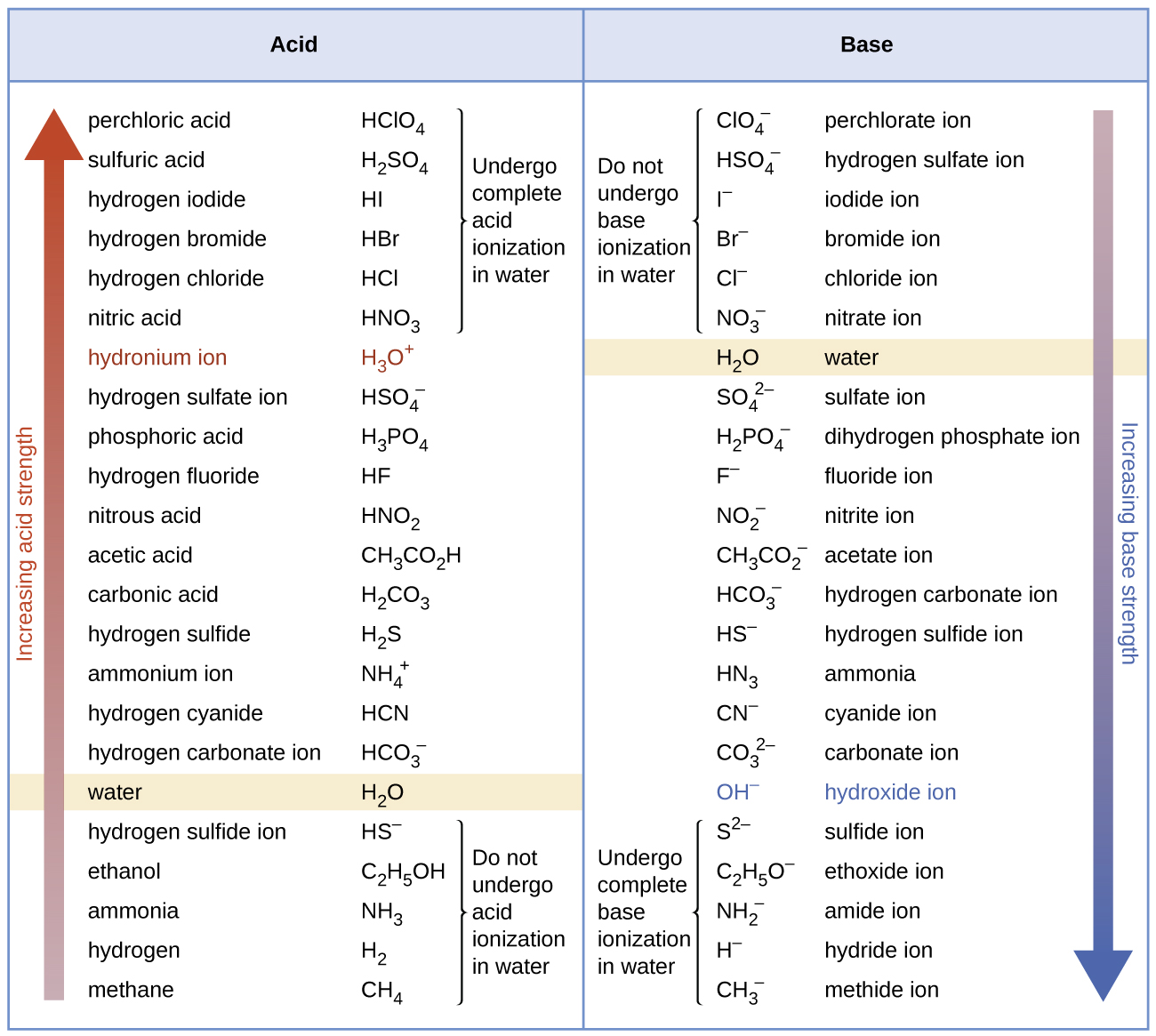
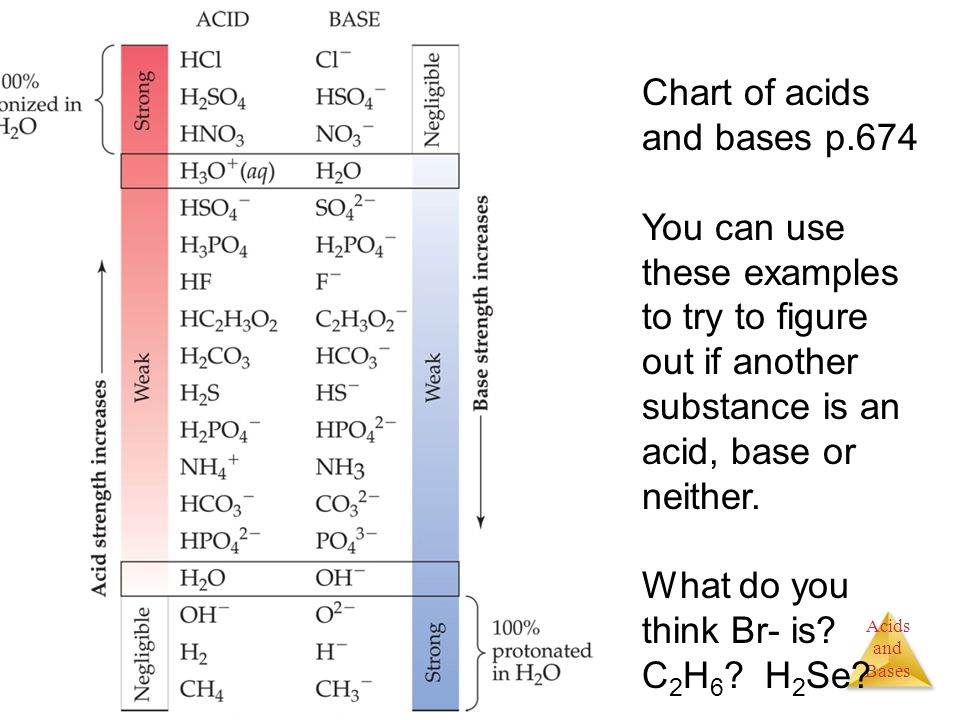
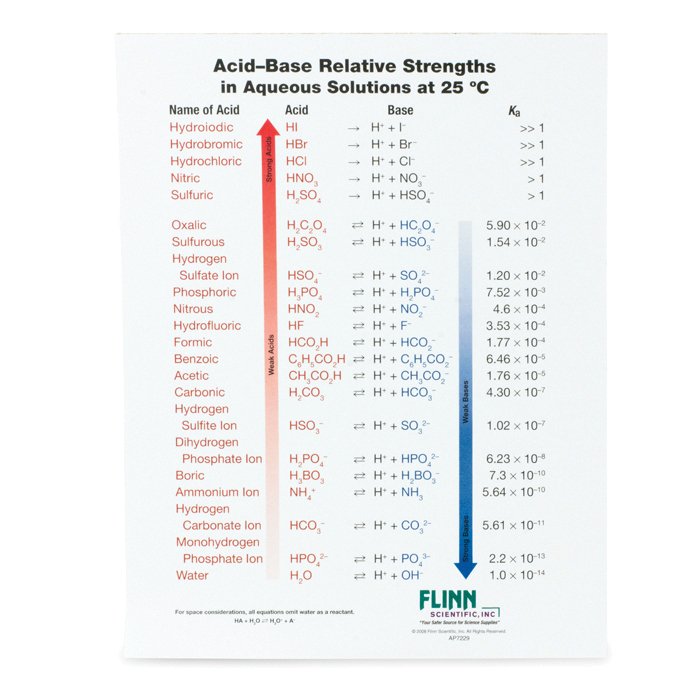
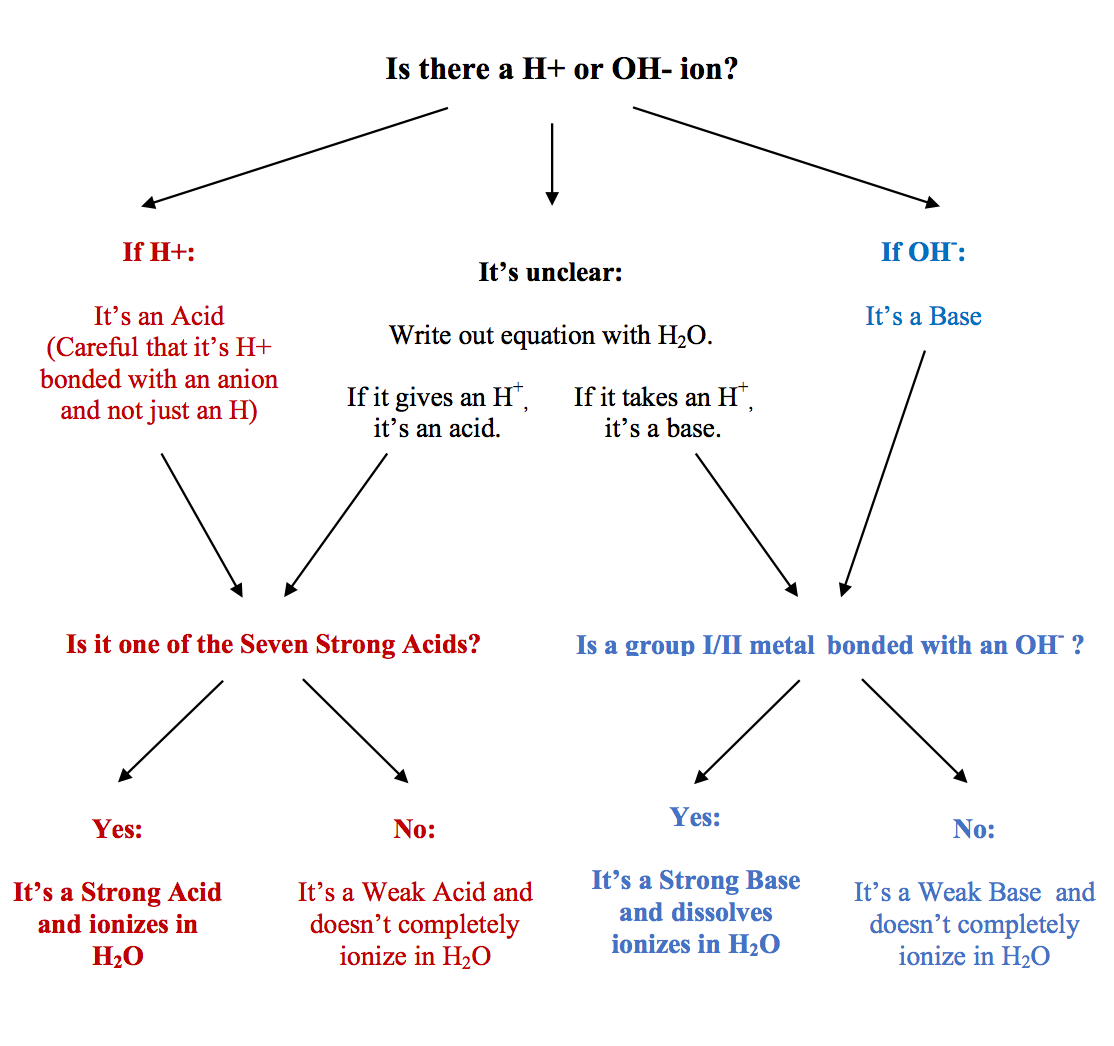
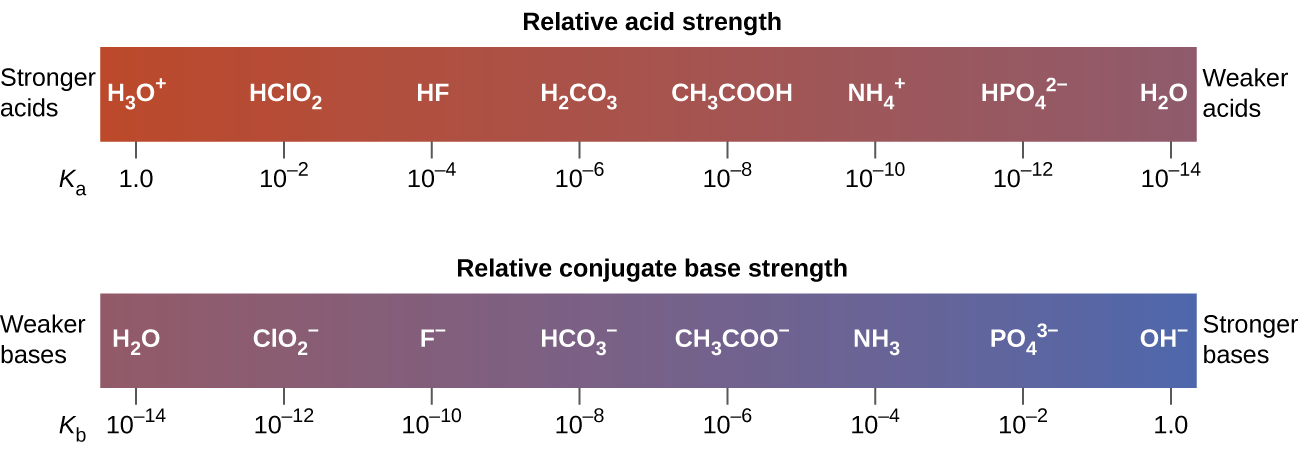
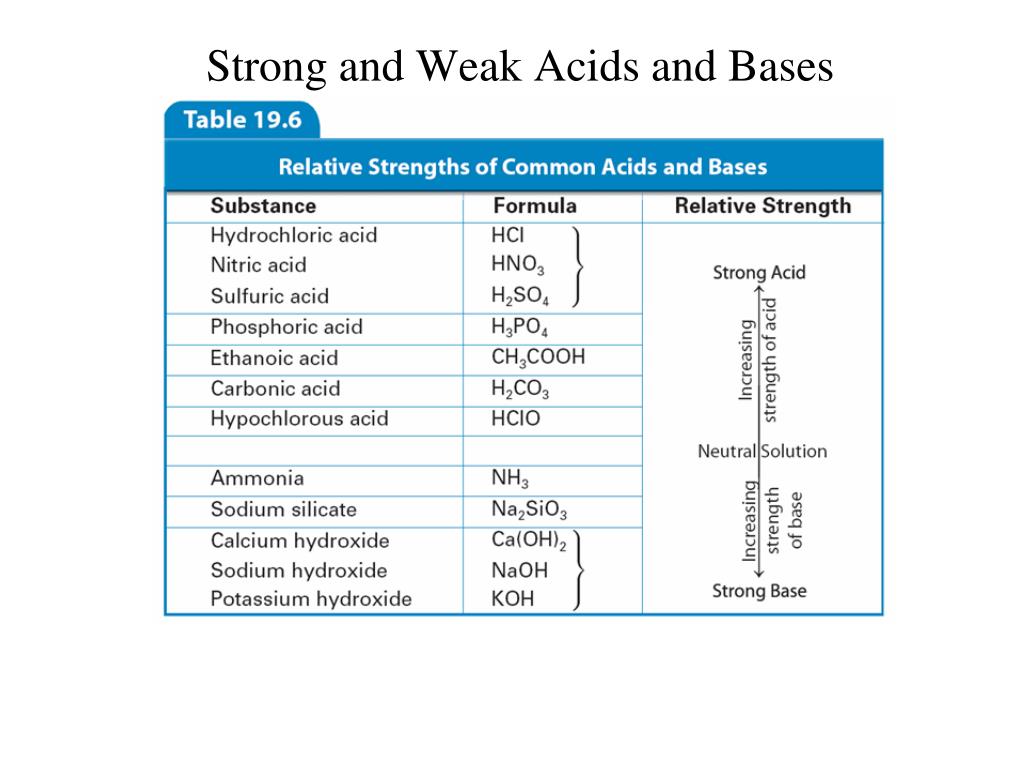
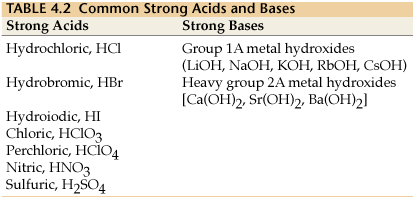
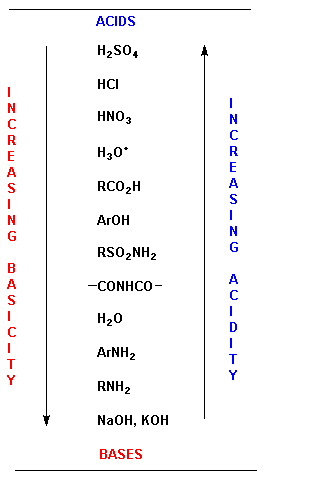
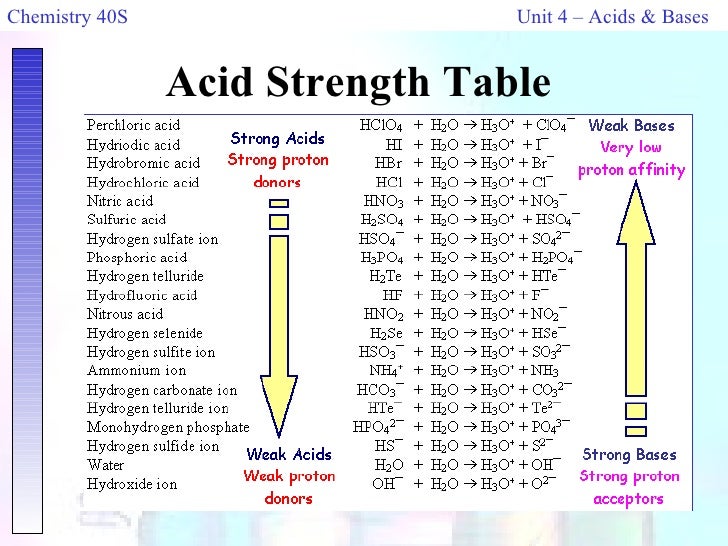
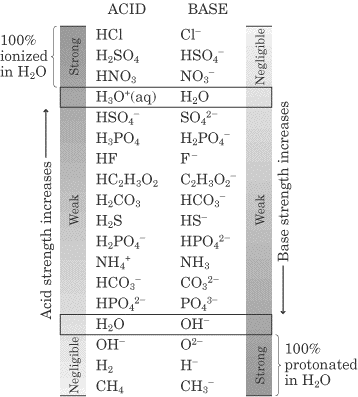
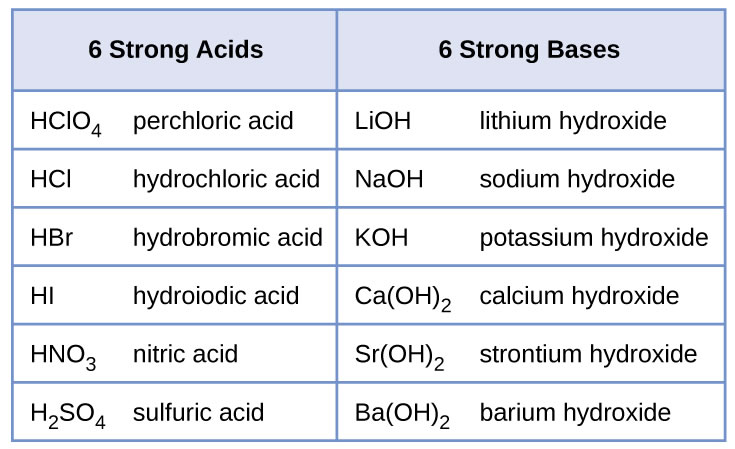
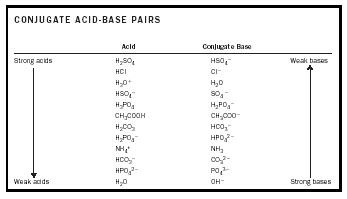
How to use the acid base chart strong acids are listed at the top left hand corner of the table and have ka values 1 acids with a ka value less than one are considered weak and get weaker as we move to the bottom of the table.
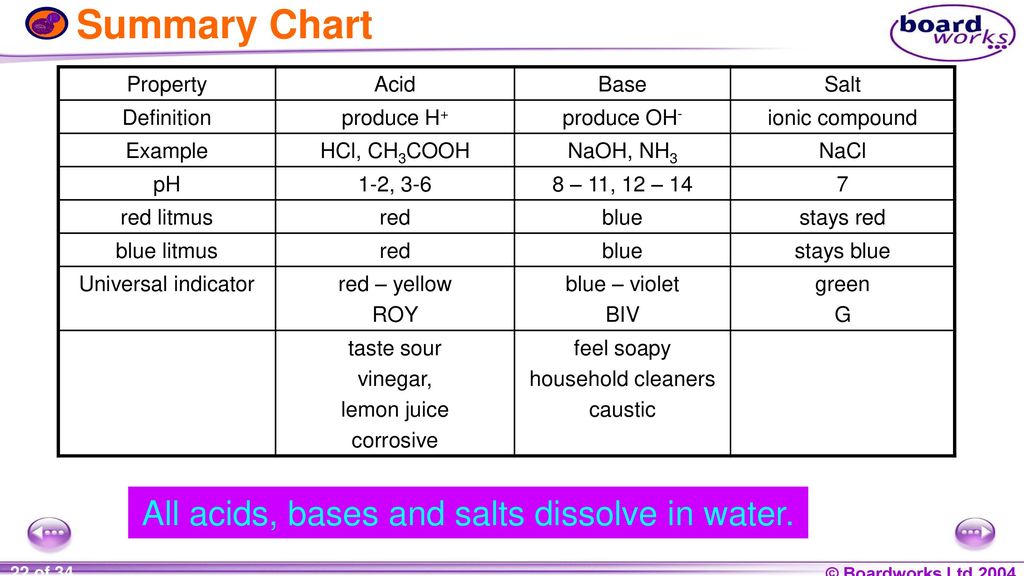
Acids and base chart. Metal ions with the highest affinities for hard bases are hard acids whereas metal ions with the highest affinity for soft bases are soft acids. Find name molecular formula strength and reagent volume for your dilution. Bases are the chemical opposite of acids.
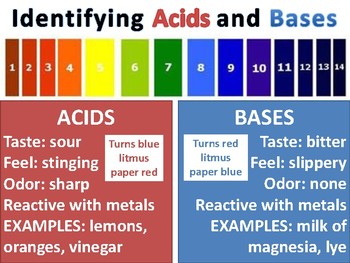
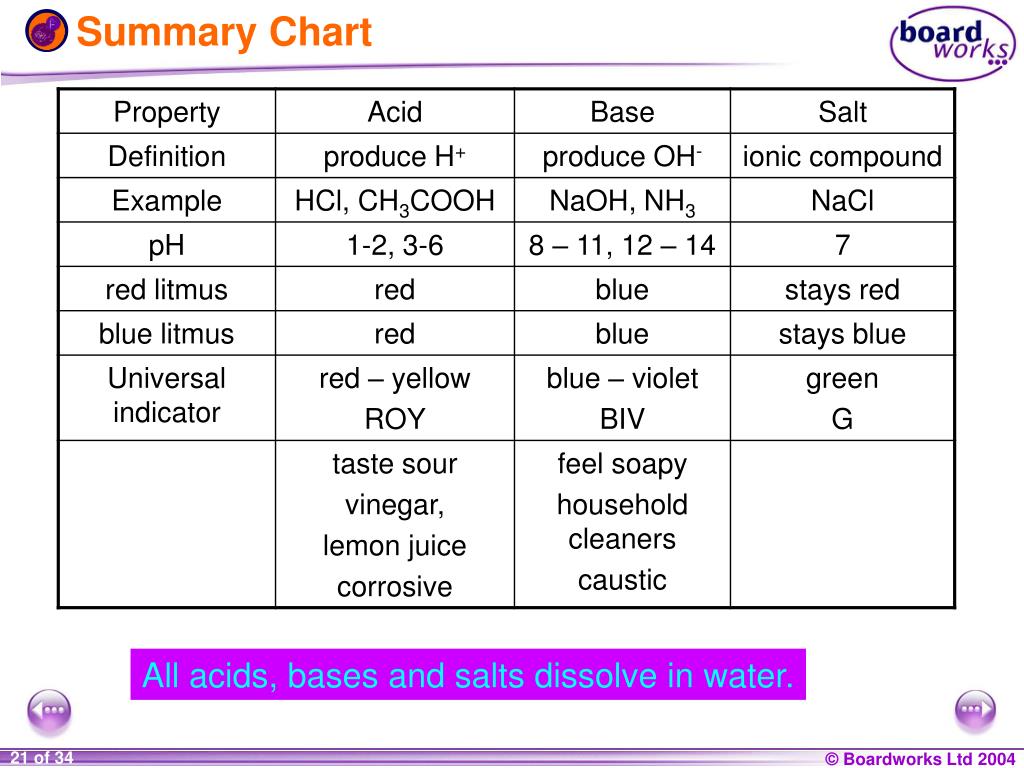
Chemical elements periodic table. Complete list of bases. A base is a substance that accepts hydrogen ions.
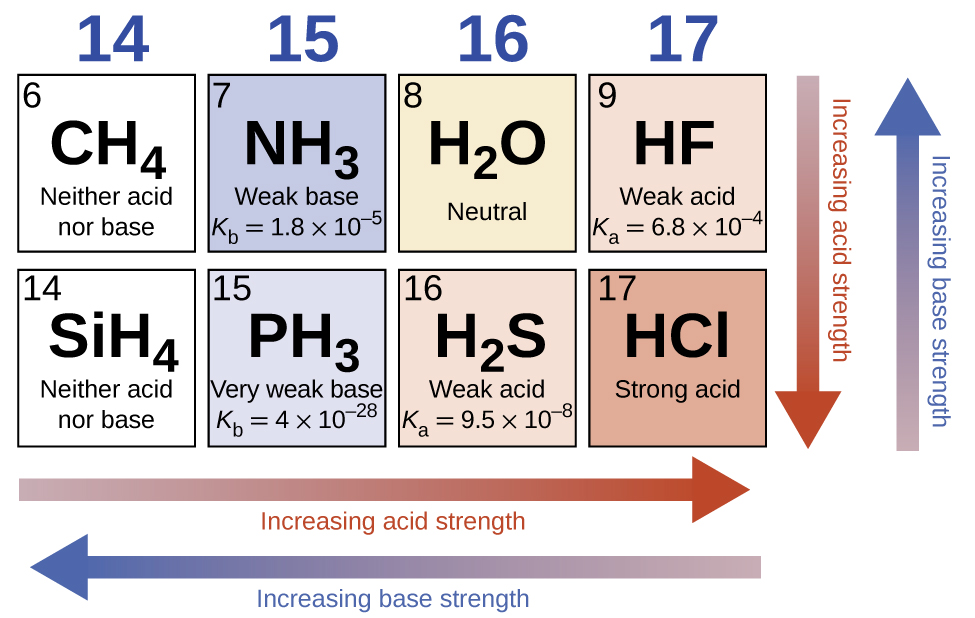
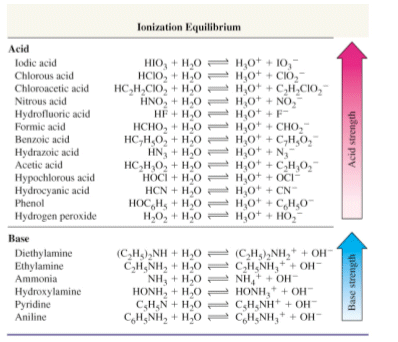
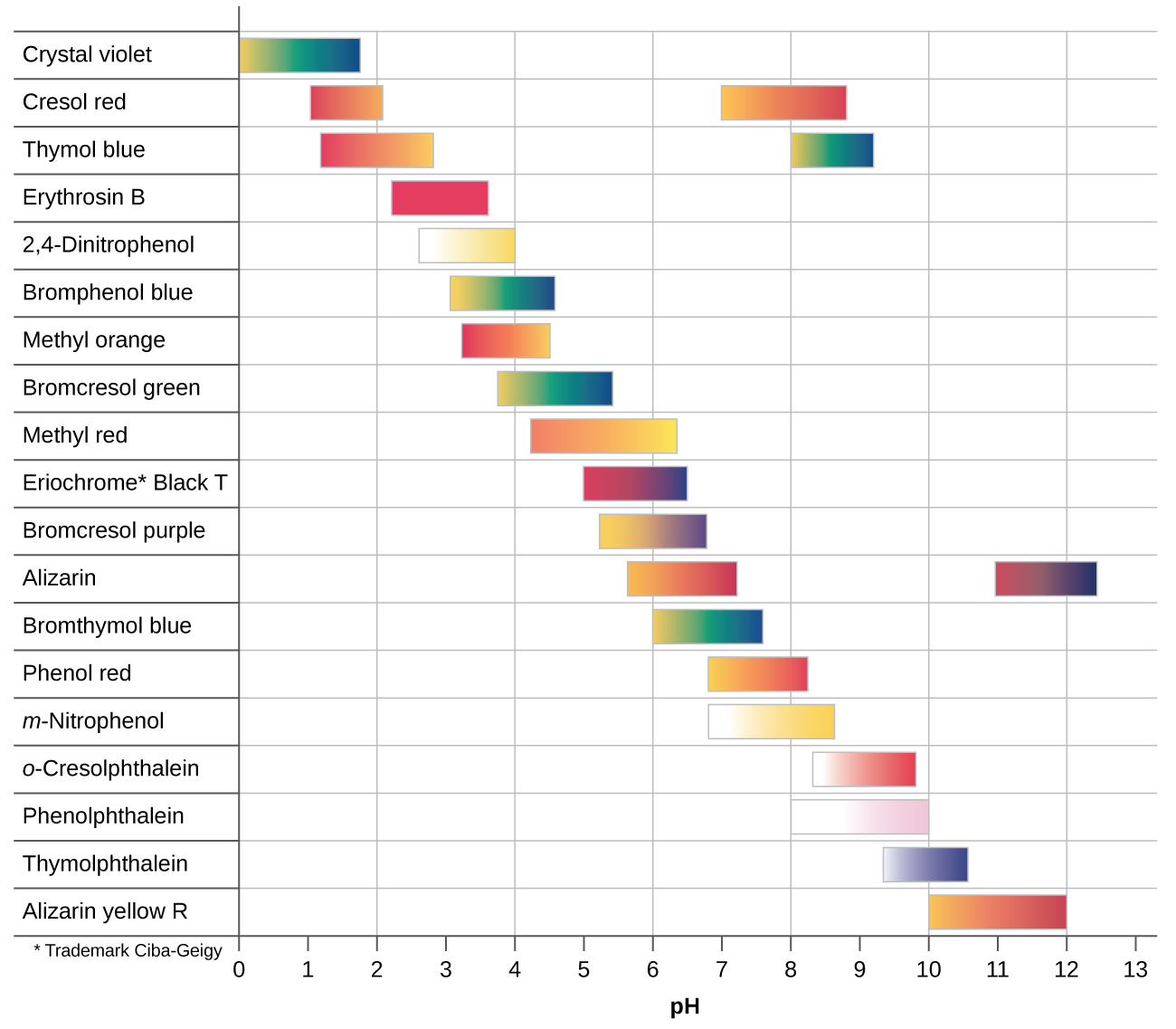
Some examples of hard and soft acids and bases are given in table pageindex 1. The undissociated form of the indicator is a different color than the iogenic form of the indicator. An acid base indicator is a weak acid or a weak base.
Strong acids are listed at the top left hand corner of the table and have ka values 1 2. Now there are more hydrogen ions than hydroxide ions in the solution. Bh 3 ch 3 2 s h 3 b s ch 3 2 cao co 2 caco 3 becl 2 2 cl becl 42.
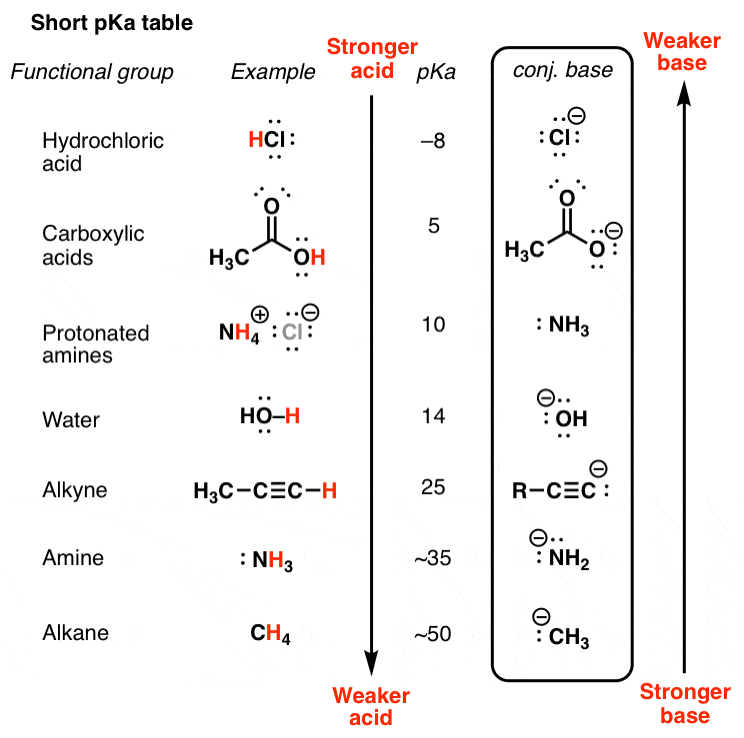
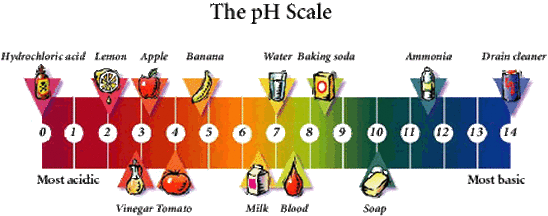
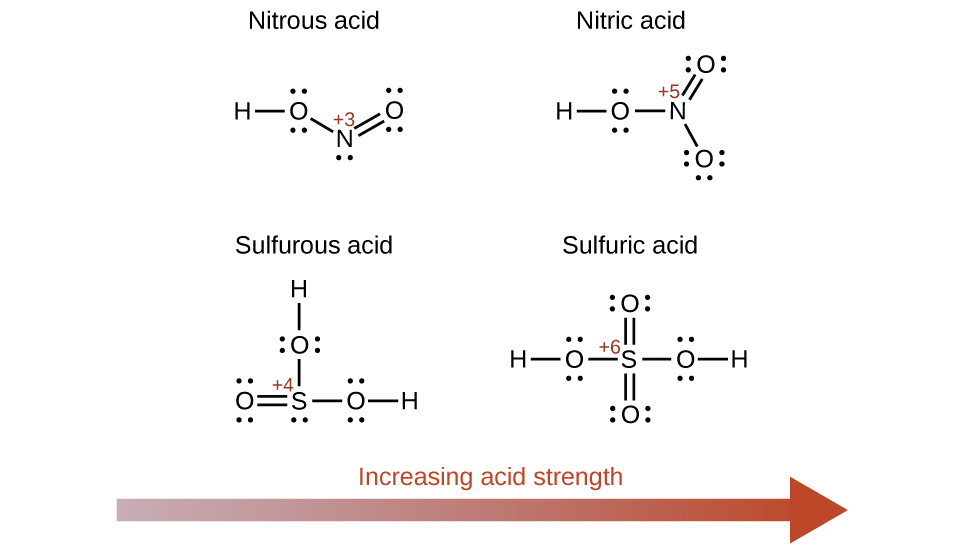
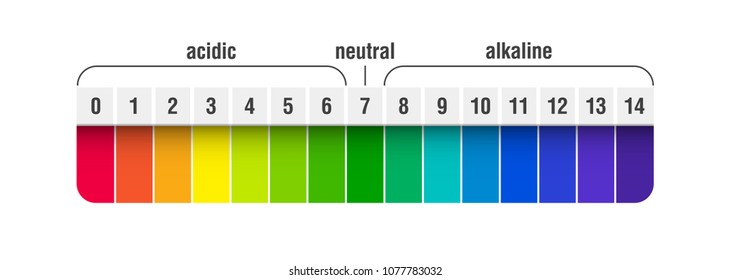
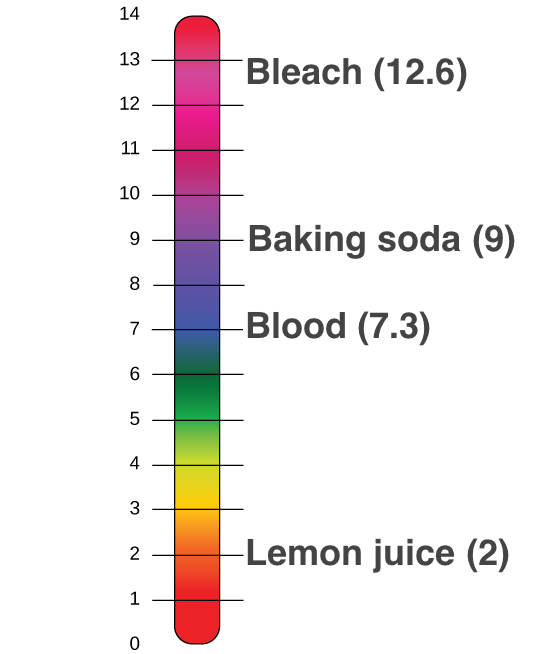
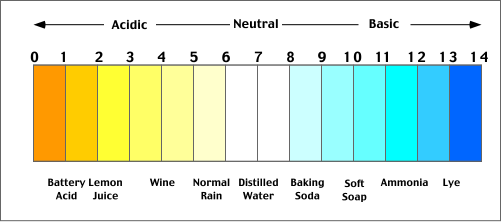
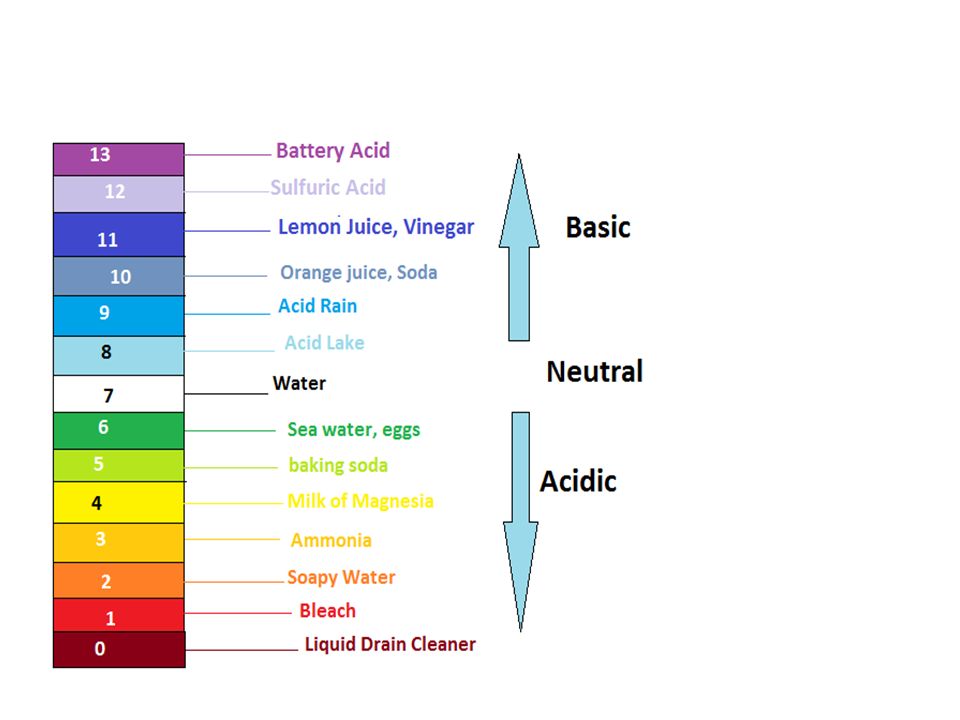
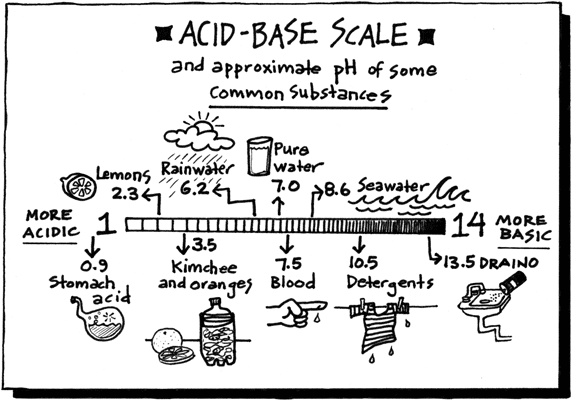
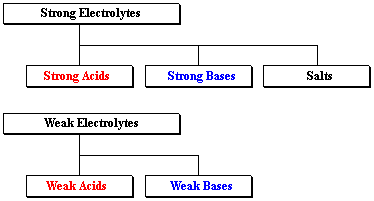
List of common organic acids. When a base is dissolved. A ph less than 7 0.
An indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration but rather color change occurs over a range of hydrogen ion concentrations. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. An acid is a substance that donates hydrogen ions.
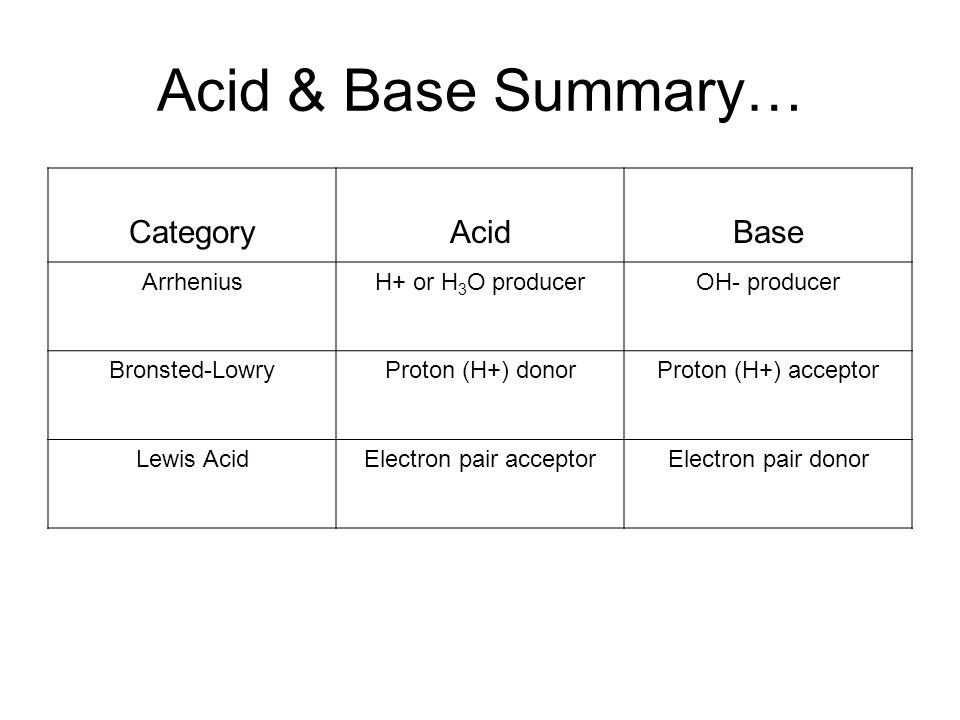
Our common acids and bases concentration reference chart allows you to easily prepare chemicals in a 1 normal solution. This kind of solution is acidic. There are many definitions which differentiate the substances as acid and base but the most accepted are the arrhenius theory bronsted lowry theory and the lewis theory of acid base.
The strong bases are listed at the bottom right of the table and get. Acids and bases are one of the most important parts of chemistry but also play their significant role in another field of science. Because of this when an acid is dissolved in water the balance between hydrogen ions and hydroxide ions is shifted.






























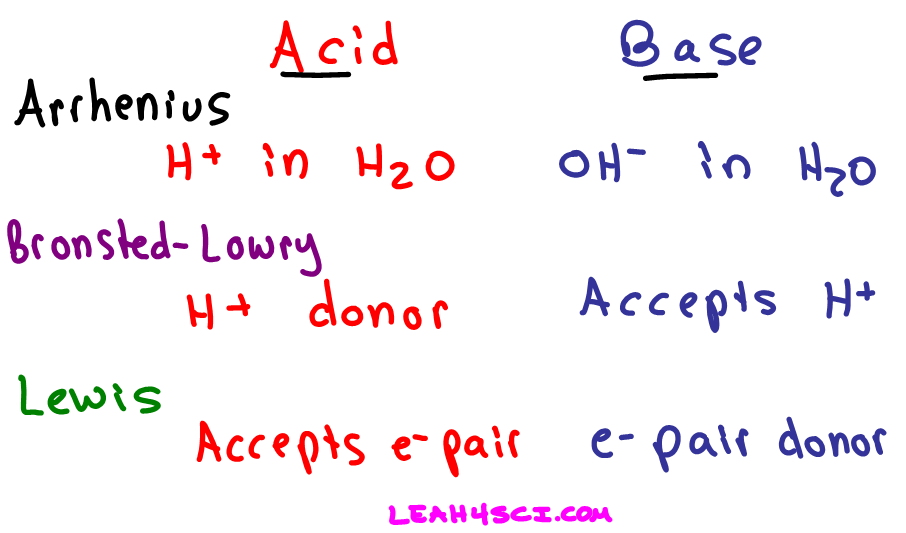


:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)










/GettyImages-680790165-5897802f3df78caebcf554a0.jpg)



